Aktuelles
-
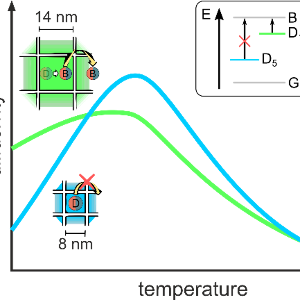 Neues Paper in Advanced Energy Materials veröffentlicht
Neues Paper in Advanced Energy Materials veröffentlichtDas ist das Ergebnis einer Kollaboration mit der Gruppe um Maksym Kovalenko (ETH Zürich) zu dem Thema Exzitonendiffusion in Nanokristallanordnungen.
-
 Neues Gruppenmitglied
Neues GruppenmitgliedBastian Schubert startet ein Praktikum in unserer Gruppe.
-
 Neuster Doktor der Arbeitsgruppe
Neuster Doktor der ArbeitsgruppeMichael Lichtenegger verteidigte erfolgreich seine Dissertation "Exciton Diffusion in Perovskite Nanocrystal Assemblies"




